Abstract
Fever-like hyperthermia is known to stimulate innate and adaptive immune responses. Although pyrogenic cytokines can stage the ground for immune defense (e.g. during the mobilization of lymphocytes to the lymphoid organs) hyperthermia per se is able to activate immune-competent cells and to induce a specific immunophenotype and gene expression profile. Hyperthermia-induced immune stimulation is also accompanied with, and likely conditioned by, changes in the cell metabolism and, in particular, mitochondrial metabolism is now recognized to play a pivotal role in this context, both as energy supplier and as signaling platform. In this study we asked if challenging human monocyte-derived dendritic cells with a relatively short-time thermal shock in the fever-range, typically observed in humans, caused alterations in the mitochondrial oxidative metabolism. We found that following hyperthermic stress (3 h exposure at 39°C) TNF-alpha-releasing dendritic cells undergo rewiring of the oxidative metabolism hallmarked by decrease of the mitochondrial respiratory activity and of the oxidative phosphorylation and increase of lactate production. Moreover, enhanced production of reactive oxygen and nitrogen species and accumulation of mitochondrial Ca2+ was consistently observed in hyperthermia-conditioned dendritic cells and exhibited a reciprocal interplay. The hyperthermia-induced impairment of the mitochondrial respiratory activity was (i) irreversible following re-conditioning of cells to normothermia, (ii) mimicked by exposing normothermic cells to the conditioned medium of the hyperthermia-challenged cells, (iii) largely prevented by antioxidant and inhibitors of the nitric oxide synthase and of the mitochondrial calcium porter, which also inhibited release of TNF-alpha. These observations combined with gene expression analysis support a model based on a thermally induced autocrine signaling, which rewires and sets a metabolism checkpoint linked to immune activation of dendritic cells.
Figure Legend.
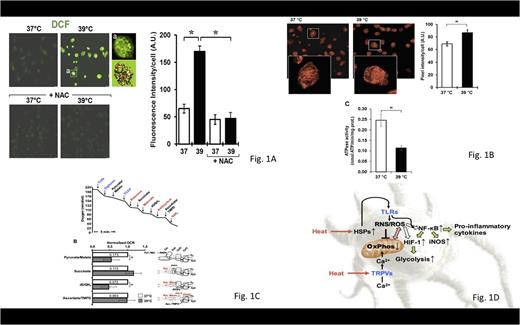
Fig. 1A Effect of mild hyperthermic treatment of MoDCs on reactive oxygen species production in live cells and respiratory activity
Fig. 1B Imaging of mitochondrial membrane potential in live cells and measurement of Complex V activity
Fig. 1C Effect of mild hyperthermic treatment of MoDCs on the activities of specific segments of the mitochondrial respiratory chain
Fig. 1D Working model of the effect of mild hyperthermic conditioning of MoDCs on cell metabolism and pro-inflammatory activation
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal